Noncompetitive affinity assays of glucagon and amylin using mirror-image aptamers as affinity probes
Abstract
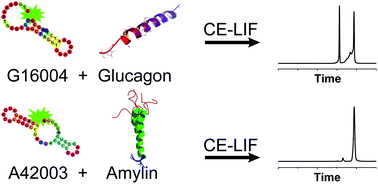
The ability to detect picomolar concentrations of glucagon and amylin using fluorescently labeled mirror-image aptamers, so-called Spiegelmers, is demonstrated. Spiegelmers rival the specificity of antibodies and overcome the problem of biostability of natural aptamers in a biological matrix. Using Spiegelmers as affinity probes, noncompetitive capillary electrophoresis affinity assays of glucagon and murine amylin were developed and optimized. The detection limit for glucagon was 6 pM and for amylin was 40 pM. Glucagon-like peptide-1 and -2 did not interfere with the glucagon assay, while the amylin assay showed cross-reactivity to calcitonin gene related peptide. The developed assays were combined with a competitive immunoassay for insulin to measure glucagon, amylin, and insulin secretion from batches of islets after incubation with different glucose concentrations. The development of these assays is an important step towards incorporation into an online measurement system for monitoring dynamic secretion from single islets.

 Please wait while we load your content...
Please wait while we load your content...