Halogen mediated voltammetric oxidation of biological thiols and disulfides
Abstract
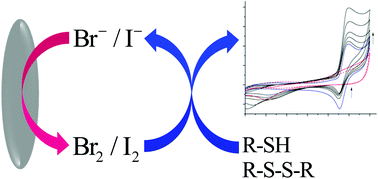
The electrochemical generation of the halides, bromine and iodine, in the presence of biologically relevant organosulfur is demonstrated to result in an analytically useful response. In the case of the iodide/iodine redox couple only the thiol causes an increase in the electrochemical oxidative peak current. Conversely, the formed bromine may catalytically oxidise both thiols and disulfides. Hence, the differing reactivities of the halide ions readily allow discrimination between the closely related thiol and disulphide species. For all of the organosulfur species investigated (glutathione, cysteine and homocysteine) micromolar limits of detection are attainable. In the case of the bromine mediated oxidation this sensitivity at least partially arises from the large catalytic amplification, such that, for each disulphide molecule up to ten electrons may be transferred. Ultimately this bromine oxidation results in the formation of the sulfonate species. For the iodine mediated oxidation of the thiols the oxidation proceeds no further than to the formation of the associated disulfide.
 Please wait while we load your content...
Please wait while we load your content...