Formation of nanoscopic CaF2via a fluorolytic sol–gel process for antireflective coatings†
Abstract
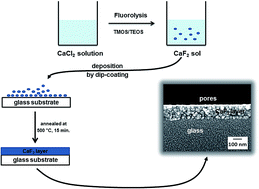
The synthesis of nanoscopic calcium fluoride was performed by the fluorolytic sol–gel process. Antireflective coatings of CaF2 were prepared from sols obtained by the reaction of CaCl2 with HF and subsequent dip coating. The addition of tetramethyl orthosilicate (TMOS) or tetraethyl orthosilicate (TEOS) after fluorination promotes the formation of transparent sols. The formation and crystallisation of CaF2 nanoparticles was studied by 19F liquid and solid state NMR spectroscopy, dynamic light scattering (DLS) and X-ray powder diffraction (XRD). The morphology of a CaF2-film was analysed by high resolution scanning electron microscopy (HR-SEM) and the mechanical stability of a CaF2-film was evaluated by the Crockmeter test using both felt and steel wool. The refractive index for a CaF2-film was measured by ellipsometry. The synthesis of CaF2 nanoparticles derived from CaCl2 is a good way to achieve porous antireflective coating layers.

 Please wait while we load your content...
Please wait while we load your content...