Enhanced electron mobility in crystalline thionated naphthalene diimides†
Abstract
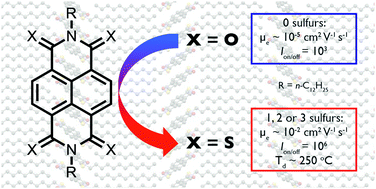
A series of five thionated naphthalene diimides (NDIs) with linear alkyl chains was synthesized and the optoelectronic, self-assembly, and device properties were studied. When tested in organic thin-film transistors, the electron mobilities of the thionated derivatives are three orders of magnitude higher than the non-thionated parent analogue, with the highest mobility measured for cis-S2 (μmax = 7.5 × 10−2 cm2 V−1 s−1). In contrast to branched chain PDIs and NDIs, the electron mobility does not increase appreciably with degree of thionation, and the average mobilities are quite consistent ranging from 3.9 × 10−2 to 7.4 × 10−2 cm2 V−1 s−1 for one to three sulfurs.

 Please wait while we load your content...
Please wait while we load your content...