Abstract
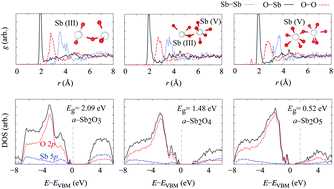
The amorphous phases of antimony oxide at three different oxidation levels have been investigated by ab initio molecular-dynamics calculations using a simulated ‘melt-quench’ approach. The atomic and electronic structures of the amorphous phases are analyzed and compared to their crystalline counterparts. The amorphous structure of the trioxide (a-Sb2O3) resembles the β-phase of the crystalline form (valentinite), in agreement with previous observations. In the relaxed athermal structure, however, more senarmontite-like structural features are apparent. The phase diagram of the amorphous phases with respect to the oxygen chemical potential has been calculated within the framework of ab initio thermodynamics. At elevated oxidation levels, the resulting tetraoxide and pentoxide materials show distinct variation in electronic structure compared to the trioxide, with the electronic density of states indicating a narrowing of the electronic gap with increasing oxidation level.

 Please wait while we load your content...
Please wait while we load your content...