Thermodynamic stability of lead-free alkali niobate and tantalate perovskites
Abstract
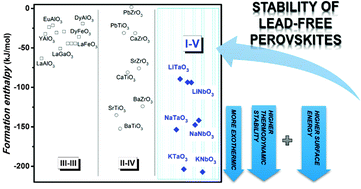
Lead-free niobates and tantalates currently form some of the most promising groups of ferroelectrics, piezoelectrics and related materials, with important applications for the next generation of lead-free sensors, actuators and microelectromechanical systems (MEMs). In view of their importance, the enthalpies of formation from binary oxide components at 25 °C, measured by high temperature oxide melt solution calorimetry of a set of alkali tantalates and niobates with perovskite-like structures, LiTaO3, LiNbO3, NaTaO3, NaNbO3 and KNbO3, are reported to be −93.74 ± 1.77, −93.44 ± 1.48, −147.35 ± 2.46, −141.63 ± 2.27 and −207.12 ± 1.74 kJ mol−1 for LiTaO3, LiNbO3, NaTaO3, NaNbO3 and KNbO3, respectively. The surface energies of nanocrystalline perovskites of these alkali tantalates and niobates were experimentally determined for the first time by calorimetry. The energies of the hydrated surface are 1.04 ± 0.34, 1.21 ± 0.78, 1.58 ± 0.29, 2.16 ± 0.57 and 2.95 ± 0.59 J m−2 for LiTaO3, LiNbO3, NaTaO3, NaNbO3 and KNbO3, respectively. The stability of the lead-free perovskites of I–V type is discussed based on their tolerance factor and acid–base chemistry. The formation enthalpy becomes more exothermic (higher thermodynamic stability) and the surface energy increases (greater destabilization for a given particle size) with the increase in the ionic radius of the A-site cations (Li, Na and K) and with increase in the tolerance factor. These correlations provide key insights into how lead-free niobates and tantalates behave during synthesis and processing; i.e. they explain, for example, why KNbO3 and KTaO3 nanoparticles are thermodynamically more reactive than their Li and Na counterparts. This understanding will facilitate the development of optimized processing techniques and applications.

 Please wait while we load your content...
Please wait while we load your content...