New highly efficient electrochemical synthesis of dispersed Ag2O particles in the vicinity of the cathode with controllable size and shape†
Abstract
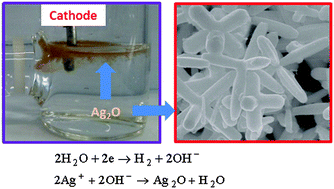
Silver(I) oxide (Ag2O) hexapod particles were electrochemically synthesized with high efficiency in an aqueous solution of silver nitrate and sodium sulfate. The generation of dispersed Ag2O particles in the vicinity of the cathode was explained by a two-step reaction mechanism involving the reduction of water, 2H2O + 2e− → H2 + 2OH−, followed by the precipitation of Ag2O particles, 2Ag+ + 2OH− → Ag2O + H2O. The applied potential played an important role in controlling the size and yield of the Ag2O hexapods. The average size of the hexapod particles was adjusted from 1.84 down to 0.60 μm by increasing the applied potential from 4 to 10 V. Furthermore, the ratio of the Ag2O/Ag that was produced was determined to be ca. 23 when the applied potential was increased up to 10 V. On the other hand, the morphological change exhibited by the Ag2O particles was determined to be a function of the silver ion concentration, which is proportional to the growth rate. The Ag2O hexapods formed as a consequence of fast growth along the equivalent 〈100〉 directions.

 Please wait while we load your content...
Please wait while we load your content...