A diethylaminophenol functionalized Schiff base: crystallization-induced emission-enhancement, switchable fluorescence and application for security printing and data storage†
Abstract
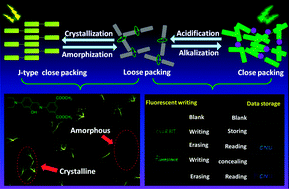
In this study, we report the synthesis and photoluminescence (PL) behaviour of a new luminogen, SBOH, a diethylaminophenol functionalized Schiff base. SBOH possesses electron donor (D) and acceptor (A) units, showing twisted intramolecular charge transfer (TICT) properties. Interestingly, SBOH is weakly emissive in the amorphous phase but becomes highly emissive upon crystallization as confirmed by spectroscopic methods and fluorescence microscopy; in other words, SBOH is crystallization-induced emission-enhancement (CIEE)-active. Similar to the typical CIEE luminogens, the emission of SBOH is also sensitive to molecular packing modes. The weak emission of amorphous SBOH can be switched on via a deprotonation process caused by strong alkali. Meanwhile, exposure to acetic acid vapour rapidly switches the emission to the “off” state through re-protonation. The protonation and deprotonation processes display good reversibility. This deprotonation process is further confirmed by 1H NMR analysis and UV-vis spectroscopy. By taking advantage of the stimuli responsive fluorescence, a convenient and efficient technology for security printing and data storage is designed.

 Please wait while we load your content...
Please wait while we load your content...