Novel s-tetrazine-based dyes with enhanced two-photon absorption cross-section†
Abstract
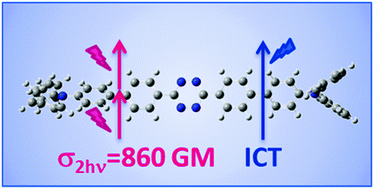
This paper reports the synthesis and the linear and non-linear absorption properties of a series of new tetrazine-based D–π–A–π–D and D–π–A type dyes. In these derivatives, a central tetrazine core was connected with one or two terminal triphenylamine moiety(ies) via various π-conjugated spacers. These compounds were efficiently synthesized by Stille or Suzuki–Miyaura cross-coupling as a key step. Their photophysical properties, including one-photon absorption and two-photon absorption (2PA), were investigated with special attention to structure–property relationships. Large 2PA cross-sections (>800 GM) of these tetrazine dyes were evaluated by open aperture z-scan and non-degenerate 2PA techniques. The strong 2PA of these molecules is attributed to the extended π system and to the enhanced intramolecular charge transfer between the triphenylamine donor and the center tetrazine acceptor.

 Please wait while we load your content...
Please wait while we load your content...