Controllable multicolor switching of oligopeptide-based mechanochromic molecules: from gel phase to solid powder†
Abstract
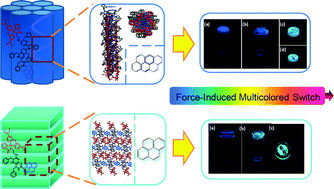
A series of molecules with pyrene and rhodamine B as color-producing mechanophores linked by different spacers were synthesized and their mechanochromic properties were studied. Interestingly, we found that the molecule with diphenylalanine as a linker (PHE-2) showed a sequential multicolored switch from deep blue to bluish green and further to a reddish color, which was associated with the phase change from gel to xerogel as the solvent evaporated, and to a solid powder triggered by grinding. However, the gel and xerogel of the molecule with the pentaphenylalanine linker (PHE-5) exhibited the same deep blue color that switched to bluish green and further to a reddish powder by virtue of continuously grinding the xerogel sample in situ. The multicolored switching of PHE-2 and PHE-5 was realized by the variation of the self-assembled structures, which induced the transition of the pyrene excimers from excimer 1 (deep blue) to excimer 2 (bluish green), and by the chemical reaction of rhodamine B from a spirolactam to a ring-opened amide (red). From the experimental results, we may conclude that the crucial point for controlling and tuning the tricolored fluorescent switch of this system is to constrict the pyrene excimer in an overlapped packing mode, which can be achieved (1) by controlling the molecular structure; and (2) by confining the excimers of pyrene in a restricted environment.

 Please wait while we load your content...
Please wait while we load your content...