The fluorescence properties and aggregation behavior of tetraphenylethene–perylenebisimide dyads†
Abstract
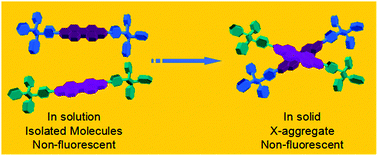
Two tetraphenylethene (TPE) modified perylenebisimides (PBIs) were synthesized through linking the TPE moieties to the PBI core at the imide positions. Theoretical calculations predict that, in both of the mono-TPE and di-TPE substituted derivatives (i.e. TPE-N-PBI and DTPE-N-PBI), the TPE and PBI units are orthogonal to each other, and thus have no electronic conjugation. Thus the two compounds are TPE–PBI dyads rather than TPE–PBI conjugates. This property is supported by absorption and emission spectral features. In solution, aggregate and solid film, the fluorescence from both TPE and PBI subunits is evidently quenched. The underlying mechanism is the photo-induced charge transfer between the electron donor TPE and electron acceptor PBI subunits. In a highly polar tetrahydrofuran–water mixture with a large fraction of water, TPE-N-PBI can form H-aggregates. In low polar hydrophobic dichloromethane–hexane mixtures, due to the bulky size and rigidity of the TPE subunit, the dyad molecules cannot take a parallel alignment to form classical J- or H-aggregates, but have to exist in a large offset angle. Consequently, X-aggregates are formed, which was confirmed by the absorption features, morphological observations and comparative investigation of the reference compound.

 Please wait while we load your content...
Please wait while we load your content...