Molecular recognition directed supramolecular control over perylene-bisimide aggregation resulting in aggregation induced enhanced emission (AIEE) and induced chiral amplification†
Abstract
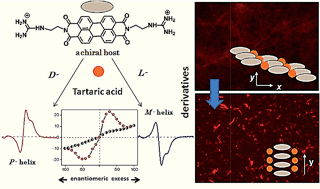
Recognition directed spontaneous assembly formation has been used to build-up a sensory system with perylene bis-guanidinium (PBG), which presented great response towards chiral guest sensing. Based on structural modification in the chiral guest molecules, PBG can selectively recognize dibenzoyl tartaric acid (DBTA) among the other tartaric acids (TA), resulting in an explicit read-out of molecular information via the generation of characteristic induced circular dichroism spectra for the D- and L-enantiomers. The binding ability depends only on the substituents in the TA and the local guidance plays an important role in structure formation. The bulky substituents in TA can lead to induced chirality in the cofacial perylene stacks by generating an active angle between the successive transition dipoles. The induced helical sense was found to exhibit efficient chiral amplification with a sigmoidal change (i.e. according to a sergeants-and-soldiers effect) in the enantiomeric excess plot. The diversity in physical properties was further examined by the preparative method. Compared to the simple mixing method, the heating–cooling method is able to show aggregation induced fluorescence enhancement, which was used to fabricate highly efficient fluorescent solid in a supramolecular manner from a perylene-based dye.

 Please wait while we load your content...
Please wait while we load your content...