Switching of the triplet excited state of the C60-dimethylaminostyryl BODIPY dyads/triads†
Abstract
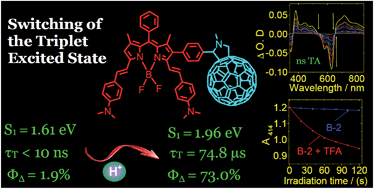
In order to switch the triplet excited states in organic compounds, dimethylaminostyryl BODIPY-C60 dyads and triads were prepared. The triplet excited states of the compounds were switched with acid/base, and the mechanism was studied with nanosecond time-resolved transient difference absorption spectroscopy. The visible light-harvesting BODIPY antennas are the electron or singlet energy donor, whereas C60 moiety is the electron/singlet energy acceptor, as well as the spin converter to produce triplet excited states. Our strategy of triplet state switching is to control either the photoinduced electron transfer (PET) or the singlet state energy transfer (EnT) from the antenna to C60 moiety by protonation of the dimethylaminostyryl BODIPY unit. Population of the triplet state was observed for the dyads with mono(4-dimethylaminostyryl) substituents on BODIPY antenna in nonpolar solvent such as toluene (τT = 168.6 μs). Formation of the charge transfer state (CTS) in polar solvent quenches the triplet excited state (τT < 10 ns). In the presence of acid, the dimethylaminostyryl BODIPY moiety is protonated, thus the electron transfer (ET) was inhibited. The cascade acid-activated EnT and the intersystem crossing (ISC) of C60 produce the triplet excited state. For the dyad and the triads with bis(4-dimethylaminostyryl) substituents on BODIPY antenna, the antenna S1 state energy level is lower than the S1 state energy level of C60; thus, no EnT to C60 exists, and no triplet state was produced upon selective excitation into the BODIPY moiety. With protonation of the amino styryl substituents, the S1 state energy level of the antenna is promoted to be higher than S1 state of C60 moiety, and as a result EnT is activated and triplet state is produced. In all the compounds, the triplet excited state is localized on the dimethylaminostyryl BODIPY moiety and not on the C60 moiety. The triplet state switching was conveyed to the singlet oxygen (1O2) photosensitizing ability of the compounds, and the variation of the singlet oxygen quantum yield, ΦΔ, is from 1.9% to 73%.

 Please wait while we load your content...
Please wait while we load your content...