Effects of aromatic spacers on film morphology and device memory performance based on imidazole–π–triphenylamine derivatives†
Abstract
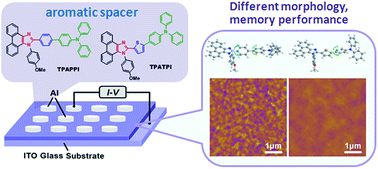
Two imidazole–π–triphenylamine derivatives TPAPPI and TPATPI, connected via different aromatic spacers (i.e., phenyl or thienyl), were synthesized. The photophysical and electrochemical properties, and memory behaviors of the two donor–π–acceptor molecules were comparatively investigated. The replacement of phenyl with thienyl leads to a much better nanoscale morphology after thermal treatment, as characterized by atomic force microscopy (AFM). Sandwich devices based on TPAPPI and TPATPI both exhibited the nonvolatile WORM characteristic but the TPATPI-based device showed a higher ON/OFF ratio and a lower switching voltage. Simulation results showed that the insertion of the thienyl spacer between the donor and acceptor moieties leads to smaller torsion between the imidazole ring and TPA moiety, which indicates a smaller charge transfer barrier and a higher extent of charge transfer (CT). This comparative study of tuning the properties of conjugated D–π–A molecules via aromatic π-spacers may be an alternative approach for the design and study of future high-performance memory devices based on new D–π–A type materials.

 Please wait while we load your content...
Please wait while we load your content...