A polyphosphoester-conjugated camptothecin prodrug with disulfide linkage for potent reduction-triggered drug delivery
Abstract
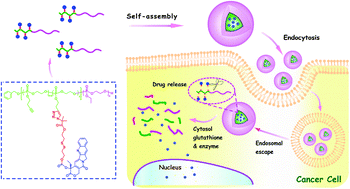
A new kind of reduction-cleavable polymer-camptothecin (CPT) prodrug has been developed, in which the polymer backbone consists of a biodegradable diblock polyphosphoester (PBYP-b-PEEP), and a modified CPT is linked onto the pendant alkynes of PBYP via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) “click” reaction to yield the polymeric prodrug, abbreviated as (PBYP-g-ss-CPT)-b-PEEP. The resulting prodrug could self-assemble into uniform prodrug micelles in aqueous solution. Since the releasable disulfide carbonate between the CPT and the polyphosphoester would be disrupted under an intracellular reducing environment, the disassociation of prodrug micelles could result in a rapid release of the CPT parent drug. The chemical structures of the intermediate polymers and a polymeric prodrug have been fully characterized by 1H NMR and FT-IR analyses, while the molecular weights and molecular weight distributions were measured by gel permeation chromatography (GPC). The self-assembly behavior of the prodrug was investigated by the fluorescence probe method, dynamic light scattering (DLS) and transmission electron microscopy (TEM) analyses. The DLS results indicated that these prodrug micelles were relatively stable in neutral pH media, but could be degraded under the reductive conditions. The in vitro drug release studies showed that the CPT release from prodrug micelles was proceeded in a glutathione (GSH)-dependent manner. A methyl thiazolyl tetrazolium (MTT) assay demonstrated that the prodrug micelles could efficiently inhibit the proliferation of HepG2 cells. In addition, the intracellular uptake of prodrug micelles could efficiently release CPT into HepG2 cells, which was observed using a live cell imaging system. All these results indicated that this GSH-responsive polymeric prodrug has high potential for reduction-triggered cancer chemotherapy.

 Please wait while we load your content...
Please wait while we load your content...