Core–shell clusters of human haemoglobin A and human serum albumin: artificial O2-carriers having various O2-affinities†
Abstract
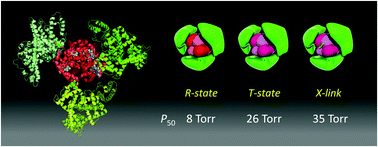
This report describes the synthesis, structure, and O2-binding properties of core–shell clusters composed of human haemoglobin A (HbA) in the centre and human serum albumin (HSA) at the periphery (HbA–HSAm) as potential O2-carriers designed as red blood cell (RBC) substitutes. Protein clusters were prepared by covalent linkages between HbA lysins and HSA cysteine-34 using a heterobifunctional cross-linker, succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate. Major products (m = 2, 3, 4) were isolated using gel filtration chromatography. The low isoelectric points (pI = 5.0–5.2) of the clusters were almost identical to that of HSA, proving the wrapping of HbA by negatively charged HSA. The 3D reconstruction of HbA–HSA3 based on transmission electron microscopy images revealed a complete triangular structure. The proposed geometries enabled us to assign a possible spatial arrangement of the HbA centre and HSA periphery. The HbA–HSAm clusters showed higher O2-affinities (P50 = 8–9 Torr) than the native HbA. The clusters prepared under N2 atmosphere showed a low O2-affinity (P50 = 26 Torr) resembling those of human RBC. Moreover, the cluster containing an αα-cross-linked HbA with bis(3,5-dibromosalicyl)fumarate showed a markedly low O2-affinity (P50 = 35 Torr). These HbA–HSAm clusters with various O2-affinities can support a new generation of RBC substitutes, which can be better tuned to play a role in O2 transport.

 Please wait while we load your content...
Please wait while we load your content...