The influence of the hydrophilic–lipophilic environment on the structure of silk fibroin protein
Abstract
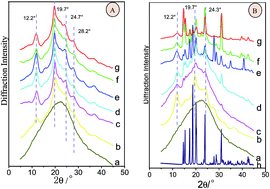
The present study examines the influence of the hydrophilic–lipophilic environment, mediated by small molecules, on the structural changes in silk protein fibroin. Small molecules mediate the various hydrophilic–lipophilic balances (HLBs) that impact the organisation of silk protein chains. Changes in the silk fibroin structure due to additives are related to the HLB value. At HLB > 10, silk fibroin primarily forms Silk I crystalline structures. Small molecules with HLB < 8.9 primarily induce the formation of Silk II crystalline structures. When 8.9 < HLB < 10, the crystalline structure of silk is related to the content of small molecules. The Silk I structure is primarily formed when the content of small molecules is low, whereas the Silk II structure is formed when the small molecule content is high. The structure of silk fibroin is maintained by regulating the HLB in the fibroin environment. This type of control for the functional design of materials may play a role in fine-tuning the biomaterial properties of silk fibroin protein.

 Please wait while we load your content...
Please wait while we load your content...