Phase transformation from hydroxyapatite to the secondary bone mineral, whitlockite
Abstract
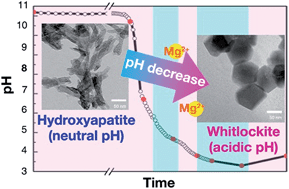
Whitlockite (WH: Ca18Mg2(HPO4)2(PO4)12) is the second most abundant mineral in hard tissues, but its precipitation mechanism or role in the body system is poorly understood. Here, using a newly discovered synthetic method for WH, we investigated the kinetic mechanism for the precipitation of WH under physiologically similar conditions, excluding any effects of toxic ions. Based on systematically classified stages in the precipitation process of WH, we monitored the transformation of calcium phosphate phases from neutral pH to acidic pH with the addition of H3PO4. The study revealed that at 70 °C, hydroxyapatite (HAP: Ca10(PO4)6(OH)2) transforms into dicalcium phosphate dihydrate (DCPD: CaHPO4·2H2O) and then into WH in the presence of Mg2+ ions as the pH decreases. The transformation process involves multiple intermediates, the stability of which depends on the cation (Ca and Mg) activities and the solution pH. WH is the most stable calcium phosphate compound below pH 4.2, whereas HAP is the most stable around neutral pH. We also found that Mg2+ ions, which are known to block the growth of HAP, can play a key role in WH formation. This study provides a new insight into the interplay of biologically important calcium phosphate compounds.

 Please wait while we load your content...
Please wait while we load your content...