Ultra-small sulphur nanoparticles configured inside a flexible organic mixed conducting network as a cathode for lithium–sulphur batteries†
Abstract
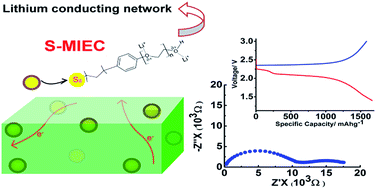
The major challenges in Li–S batteries are the formation of soluble polysulphides during the reversible conversion of S8 ↔ Li2S, large changes in sulphur particle volume during lithiation and extremely poor charge transport in sulphur. We demonstrate here a novel and simple strategy to overcome these challenges towards practical realization of a stable high performance Li–S battery. For the first time, a strategy is developed which does away with the necessity of pre-fabricated high surface area hollow-structured adsorbates and also multiple nontrivial synthesis steps related to sulphur loading inside such adsorbates. A lithiated polyethylene glycol (PEG) based surfactant tethered on ultra-small sulphur nanoparticles and wrapped up with polyaniline (PAni) (abbreviated as S-MIEC) is demonstrated here as an exceptional cathode for Li–S batteries. The PEG and PAni network around the sulphur nanoparticles serves as an efficient flexible trap for sulphur and polysulphides and also provides distinct pathways for electrons (through PAni) and ions (through PEG) during battery operation. Contrary to the cathodes demonstrated based on various carbon–sulphur composites, the mixed conducting S-MIEC showed an extremely high loading of 75%. The S-MIEC exhibited a stable capacity of nearly 900 mA h g−1 at the end of 100 cycles at a 1C current rate.

 Please wait while we load your content...
Please wait while we load your content...