High-silica, heulandite-type zeolites prepared by direct synthesis and topotactic condensation†
Abstract
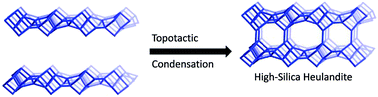
There are both natural minerals and synthetic zeolites that possess the HEU framework topology. These materials have a limited compositional range (Si/Al < 6), and the natural zeolites often contain a large amount of impurities such as Fe3+. The preparation of impurity-free HEU-type zeolites with higher Si/Al ratio could open many areas of application, particularly in catalysis. Here, we report the first high-silica HEU-type zeolite that can be prepared via two different procedures. In the first method high-silica HEU (denoted CIT-8) is prepared using a topotactic condensation mechanism (layered precursor denoted CIT-8P); CIT-8P is obtained from a low-water synthesis in fluoride media. CIT-8 prepared in this manner has a product Si/Al ratio of 9.8 ± 0.7 and a micropore volume of 0.10 cm3 g−1 (measured by nitrogen adsorption). The variable temperature powder X-ray diffraction shows that CIT-8 forms via topotactic condensation from CIT-8P along the b axis. Additionally, high-silica heulandite can be synthesized directly from a hydroxide-mediated reaction mixture (denoted CIT-8H), and has a Si/Al ratio of 6.4 ± 0.3 and a micropore volume of 0.10 cm3 g−1. Both synthesis methods produce zeolites that expand the compositional range of HEU-type zeolites. These synthetic methods allow for the addition of other heteroatoms, and titanium-containing CIT-8 is prepared as an illustrative example.


 Please wait while we load your content...
Please wait while we load your content...