The unexpected discovery of the Mg(HMDS)2/MgCl2 complex as a magnesium electrolyte for rechargeable magnesium batteries†
Abstract
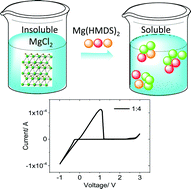
We developed a unique class of non-Grignard, aluminum-free magnesium electrolytes based on a simple mixture of magnesium compounds: magnesium hexamethyldisilazide (Mg(HMDS)2) and magnesium chloride (MgCl2). Through a reverse Schlenk equilibrium, a concentrated THF solution of Mg(HMDS)2–4MgCl2 was prepared to achieve reversible Mg deposition/dissolution, a wide electrochemical window, and a coulombic efficiency of 99%. High reversible capacities and good rate capabilities were obtained in Mg–Mo6S8 cells using these new electrolytes in tests with different rates. The unexpected high solubility of MgCl2 in the solvent of THF with the help from Mg(HMDS)2 provides a new way to develop magnesium electrolytes.

 Please wait while we load your content...
Please wait while we load your content...