Gold aerogel supported on graphitic carbon nitride: an efficient electrocatalyst for oxygen reduction reaction and hydrogen evolution reaction†
Abstract
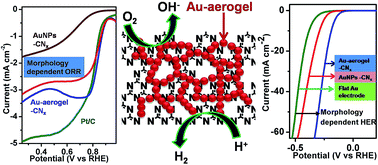
Fabrication of a high surface area interconnected porous network of metallic nanomaterials is important for their applications in various fields such as catalysis, sensors, and electrochemistry. Here we report a facile bottom up synthesis of high surface area and porous gold aerogel supported on carbon nitride sheets (CNx). The reduction of HAuCl4 in the presence of carbon nitride nanosheets using sodium borohydride and ultrasonic treatment produces gold aerogel supported on carbon nitride (Au–aerogel–CNx). When the reduction of HAuCl4 in the presence of CNx nanosheets was performed using only ultrasonication, highly dispersed ultrasmall (∼2 nm) gold nanoparticles on CNx sheets (AuNPs–CNx) were formed. The Au aerogel supported on CNx sheets was well characterized by powder X-ray diffraction, tunneling electron microscopy, selected area electron diffraction, energy dispersive X-ray spectroscopy, scanning electron microscopy, UV-visible and X-rayphotoelectron spectroscopic methods. The Au–aerogel–CNx and AuNPs–CNx composites exhibited superior electrocatalytic activity towards oxygen reduction reaction (ORR) in alkaline and acidic media. The Au–aerogel–CNx composite showed ORR onset potentials at 0.92 V and 0.43 V (vs. RHE) in 0.5 M KOH and H2SO4 solution. The four electron oxygen reduction process occurred at these supported catalysts in both alkaline and acidic media. In alkaline (KOH) medium the onset potential at Au–aerogel–CNx was more positive (∼30 mV) than that of commercial Pt/C catalyst. The composites displayed excellent methanol tolerance and comparable durability with commercial Pt/C. Furthermore, the Au–aerogel–CNx composites exhibited high catalytic activity for the hydrogen reduction reaction (HER) with a small onset potential of −30 mV and a Tafel slope of 53 mV dec−1 in acidic medium. At a small Au loading of 0.130 mg cm−2, this catalyst also exhibits a current density of 10 mA cm−2 at a low overpotential of −185 mV with excellent stability. The ORR and HER performances on porous Au–aerogel–CNx composites were better than those of the AuNPs–CNx catalyst and commercial flat gold electrode. The superior ORR and HER activities at the Au–aerogel–CNx composite originated from the unique synergistic effects between the porous Au network and carbon nitride (CNx) support.

 Please wait while we load your content...
Please wait while we load your content...