Sodiated carbon: a reversible anode for sodium–oxygen batteries and route for the chemical synthesis of sodium superoxide (NaO2)†
Abstract
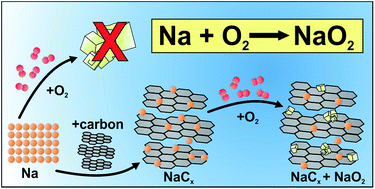
The common cell design of sodium/oxygen batteries is based on an alkali metal as the negative electrode and a carbon gas diffusion layer (GDL) as the positive (oxygen) electrode. The use of sodium metal anodes maximizes the energy capacity, but on the other hand induces undesired and often unpredictable side reactions that complicate investigations on the oxygen electrode and the overall cell chemistry. Therefore we demonstrate the function of a sodium-ion/oxygen battery by replacing sodium metal with a sodiated carbon electrode that is able to reversibly store up to q = 125 mA h g−1. We use a symmetric “all GDL” arrangement, i.e. the same carbon gas diffusion layer is used as the positive and negative electrode. Overall, this approach increases the cycle life by a factor of 5 and further decreases the sum of the charge and discharge overpotentials (η = 150 mV @ j = 200 μA cm−2), proving that current limitations of the sodium–oxygen battery are mainly determined by the metal anode rather than by the oxygen cathode. We find that sodium storage in the GDL proceeds by at least two different mechanisms which can be distinguished by their different chemical stabilities against oxygen and water. Another important finding is that NaO2 can be also synthesized chemically (rather than electrochemically) under ambient conditions from sodiated carbon and gaseous oxygen – which is interesting with respect to the competition between NaO2 and Na2O2 as discharge products.

 Please wait while we load your content...
Please wait while we load your content...