An integrated cobalt disulfide (CoS2) co-catalyst passivation layer on silicon microwires for photoelectrochemical hydrogen evolution†
Abstract
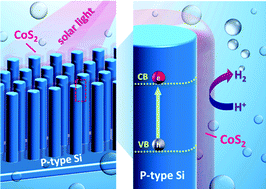
An integrated cobalt disulfide (CoS2) co-catalyst passivation layer on Si microwires (MWs) was used as a photocathode for solar hydrogen evolution. Si MWs were prepared by photolithography and dry etching techniques. The CoS2–Si photocathodes were subsequently prepared by chemical deposition and thermal sulfidation of the Co(OH)2 outer shell. The optimized onset potential and photocurrent of the CoS2–Si electrode were 0.248 V and −3.22 mA cm−2 (at 0 V), respectively. The best photocatalytic activity of the CoS2–Si electrode resulted from lower charge transfer resistances among the photoabsorber, co-catalyst, and redox couples in the electrolyte. X-ray absorption near edge structure was conducted to investigate the unoccupied electronic states of the CoS2 layer. We propose that more vacancies in the S-3p unoccupied states of the CoS2–Si electrode were present with a lower negative charge of S22− to form weaker S–H bond strength, promoting water splitting efficiency. Moreover, the CoS2 co-catalyst that completely covered underlying Si MWs served as a passivation layer to prevent oxidation and reduce degradation during photoelectrochemical measurements. Therefore, the optimal CoS2–Si electrode maintained the photocurrent at about −3 mA cm−2 (at 0 V) for 9 h, and its hydrogen generation rate was approximately 0.833 μmol min−1.

 Please wait while we load your content...
Please wait while we load your content...