Grafting alkylamine in UiO-66 by charge-assisted coordination bonds for carbon dioxide capture from high-humidity flue gas†
Abstract
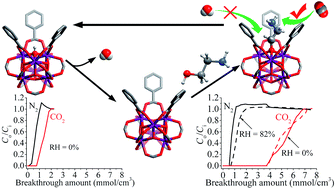
Ethanolamine (Hea) was grafted onto the pore surface of a metal–organic framework [Zr6O4(OH)4(bdc)6] (UiO-66, H2bdc = benzene-1,4-dicarboxylic acid) by a two-step post-synthetic treatment, in which UiO-66 was first dehydrated to form [Zr6O6(bdc)6], and then reacted with Hea to form [Zr6O4(OH)2(ea)2(bdc)6] (UiO-66-EA). CO2 adsorption measurements showed that UiO-66-EA exhibits a CO2 adsorption enthalpy of 66 kJ mol−1, which is much higher than that of UiO-66 (30 kJ mol−1). Solid-state 13C NMR spectra demonstrated that carbamate species are formed by the reaction of CO2 with the amine groups in UiO-66-EA. Single-component gas adsorption measurements indicated a CO2/N2 selectivity of 365 for UiO-66-EA (15 : 75 CO2/N2 at 298 K), which is about 20 times higher than the value for UiO-66 (16). Breakthrough experiments under simulated flue gases conditions (10 : 90 CO2/N2 at 313 K) showed that the N2 purification capacity of UiO-66-EA is ca. 18 times that of UiO-66. More importantly, the breakthrough performance of UiO-66-EA can be completely maintained even at high relative humidity (82%).

 Please wait while we load your content...
Please wait while we load your content...