Porous nitrogen-doped carbon-immobilized bimetallic nanoparticles as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane†
Abstract
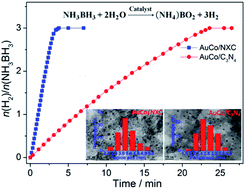
Two N-doped carbon materials, N-doped Vulcan XC-72 carbon labelled as NXC and graphite carbon nitride labelled as C3N4, were selected as supports to prepare a series of bimetallic AuM (M = Co, Ni) nanoparticles (NPs) using three different reduction ways towards mixed metal ions AuCl4− and M2+, and then the as-synthesized supported bimetallic AuM NPs were used as catalysts for hydrolytic dehydrogenation of ammonia borane (NH3BH3). All the catalysts exhibited high dispersion and small size of bimetallic NPs; however, they exhibited remarkably different catalytic activities featuring total turnover frequency (TOF) values from 6.4 to 42.1 molH2 molcat−1 min−1. Among all the catalysts, the NXC-immobilized AuCo NPs through in situ synthesis using NaBH4 and NH3BH3 as reductants exhibited the highest activity with a total TOF value of 42.1 molH2 molcat−1 min−1, which was among the highest values for Co-based catalysts ever reported for hydrolytic dehydrogenation of NH3BH3. This remarkably enhanced activity may be attributed to the synergistic effect of the N-doped NXC support and AuCo NPs and the resulting highly efficient activation of N–B bond in NH3BH3. In addition, the AuCo catalysts showed good recyclability, demonstrating that they had high stability/durability.

 Please wait while we load your content...
Please wait while we load your content...