Honeycomb-like porous iron fluoride hybrid nanostructures: excellent Li-storage properties and investigation of the multi-electron reversible conversion reaction mechanism†
Abstract
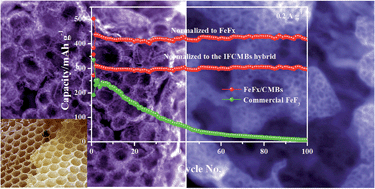
Iron fluoride cathodes with good specific energy/power performance can hardly operate durably at room temperature due to poor conductivity and sluggish kinetics. Fabricating novel hybrid nanostructures is a promising approach to obtain a fast diffusion and transport process. In this study, a porous honeycomb-like iron fluoride hybrid composite comprising iron fluoride nanocrystals (∼1–4 nm) encapsulated in separate carbon nests constructed by multi-scale pores (∼1–100 nm) was fabricated through a combination of room-temperature fluorination and a mild annealing process for the first time. The iron fluoride topochemically evolved from a smaller iron oxide nanocrystal precursor (∼2–3 nm) is closely engineered with carbon creases nested in carbon microbubbles (CMBs) which exhibit a three dimensional (3D) porous honeycomb-like network structure. As a cathode material for lithium-ion batteries (LIBs), the hybrid electrode delivers a large capacity of nearly 500 mA h g−1 at 20 mA g−1 (normalized to the composite, i.e. the capacity is calculated based on the total mass of the composite). Meanwhile, a durable cyclability of more than 500 cycles and a large rate of 10 A g−1 were also realized at room temperature. The impressive specific energy/power performance (1100 W h kg−1/224 W kg−1) which is superior to that of today's Li-ion batteries (∼380 W h kg−1/∼80 W kg−1) reveals the efficiency of the novel hybrid nanostructure in speeding up the kinetics without sacrificing the storage capability. Direct insights into the lithiation process reveal that iron fluoride firstly undergoes a mild amorphization process, and then crystallizes as γ-Fe nanocrystals after in-depth lithiation; during the de-lithiation process, γ-Fe firstly becomes amorphous due to the injection of fluorine, and subsequently evolves into double-salt-like LixFeFy nanocrystals for further fluorine enriching. Reversible conversion between C-Fe0/LiF and T-FeF2, like LixFe3+Fy with a 3 mole electron transfer, lasts for more than 100 cycles without any obvious re-distribution of the active materials.

 Please wait while we load your content...
Please wait while we load your content...