Metal–organic framework immobilized cobalt oxide nanoparticles for efficient photocatalytic water oxidation†
Abstract
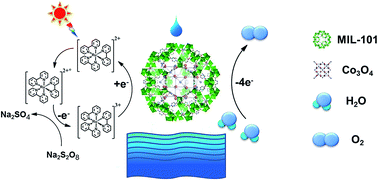
Water oxidation reactions driven by visible light play an important role in solar fuel production. Recently, catalysts based on earth abundant elements, such as cobalt oxides, have been studied extensively. Out of many factors, the catalyst particle size certainly affects the photocatalytic activity. To reduce the catalyst particle size below 5 nm without encountering agglomeration, a practical approach is to adopt a proper substrate to immobilize the catalyst nanoparticles. Herein, we utilized MIL-101, a highly porous and robust metal–organic framework (MOF), to immobilize cobalt oxide nanoparticles by a simple and facile method involving double solvent impregnation followed by a mild heat treatment. With cobalt loading in the range of 1.4–4.9 wt%, ultra small cobalt oxide nanoparticles (2–3 nm) have been successfully immobilized in the cages of MIL-101 with a good dispersion and narrow size distribution. Photocatalytic and electrochemical studies have indicated that the resultant cobalt oxide nanoparticles embedded in the MOF are highly efficient and stable water oxidation catalysts. A high turnover frequency (TOF) of 0.012 s−1 per cobalt atom and oxygen yield of 88% were obtained under the optimized conditions in the [Ru(bpy)3]2+–Na2S2O8 system. The MIL-101 support plays the roles of confining the size of catalyst nanoparticles and promoting charge transfer, leading to an enhanced photocatalytic performance.

 Please wait while we load your content...
Please wait while we load your content...