Lithium storage properties of in situ synthesized Li2FeSiO4 and LiFeBO3 nanocomposites as advanced cathode materials for lithium ion batteries†
Abstract
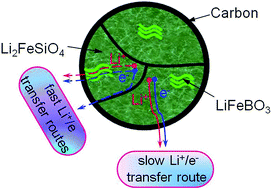
(1 − x)Li2FeSiO4·xLiFeBO3/C (x = 0, 0.02, 0.05, 0.08, 0.12, and 1.00) hetero-grains are successfully synthesized via an in situ citric acid-based sol–gel method and evaluated as cathode materials for lithium ion batteries. As a result, 0.92Li2FeSiO4·0.08LiFeBO3/C delivers an optimum discharge capacity of 251 mA h g−1 which corresponds to nearly 1.51 Li+ intercalation per molecule. Furthermore, a capacity retention of 104.5% after 5 cycles at 0.1 C is attained, and a value of 188.3 mA h g−1 (approaching 1.13 Li+ intercalation per molecule) remains even after one hundred cycles at 1 C. In particular, at a high-rate of 10 C, there is almost no capacity decline even after 500 cycles, indicating an excellent cycling stability. The greatly improved electrochemical lithium storage properties of the novel in situ hybridized materials can be attributed to the enhanced kinetics towards Li+ diffusion and electron transport compared with that of pure Li2FeSiO4/C electrodes.

 Please wait while we load your content...
Please wait while we load your content...