Safer salts for CdTe nanocrystal solution processed solar cells: the dual roles of ligand exchange and grain growth†
Abstract
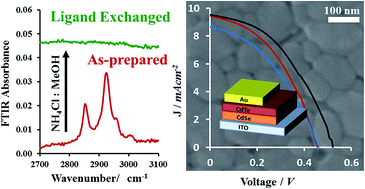
Inorganic CdSe/CdTe nanocrystals for solid-state photovoltaic devices are typically sintered into a bulk-like material after annealing in the presence of solid cadmium chloride. As in commercial CdTe devices, this salt exposure is a key component to improve device performance by promoting grain growth. However, in contrast to vapor depositions, we demonstrate that the role of the salt treatment also involves crucial ligand removal reactions, which are a unique challenge facing nanocrystal ink depositions. After testing other salts such as CdF2, CdCl2, CdBr2, CdI2 and Cd(NO3)2 for oleate ligand removal as determined by FTIR, SEM imaging of CdTe grain growth revealed the largest grains were observed from reactions with CdCl2 (142 ± 26 nm) and, to a lesser extent, CdBr2 (131 ± 19 nm). These results were used to identify cadmium-free alternatives. Trimethylsilyl chloride (28.0 ± 5.1 nm), NH4Br (75.5 ± 31 nm) and NH4Cl (136 ± 39 nm) were also tested, demonstrating comparable ligand removal and grain growth to the cadmium halides. In order to validate these observations, heterojunction photovoltaic devices were fabricated from CdSe/CdTe nanocrystals treated with non-toxic NH4Cl in place of the conventional CdCl2. Under AM 1.5G illumination, open circuit voltages (Voc), short circuit currents (Jsc) and efficiencies (η) of solar cells processed with evaporated Au and commercial ITO were found to be Voc = 0.46 ± 0.02 V, Jsc = 9.27 ± 0.6 mA cm−2, and η = 1.73 ± 0.24 demonstrating minimal differences in film morphology and device performance compared to those fabricated using cadmium chloride. Specific properties of the salts (solubility, reactivity, melting point and the identity of both the cation and the anion) were found to have a profound impact on grain growth and consequently device performance, suggesting the need for further investigation of additional non-toxic metal halide salts for this reaction.

 Please wait while we load your content...
Please wait while we load your content...