Tetrazole substituted polymers for high temperature polymer electrolyte fuel cells†
Abstract
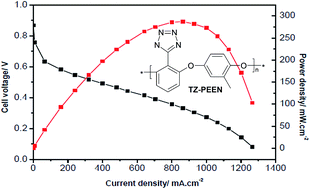
While tetrazole (TZ) has much lower basicity than imidazole and may not be fully protonated in the presence of phosphoric acid (PA), DFT calculations suggest that the basicity of TZ groups can be increased by the introduction of a 2,6-dioxy-phenyl-group in position 5 of TZ. This structure allows hydrogen bonds between TZ protons and ether oxygen atoms, and thereby establishes a resonance stabilised, co-planar structure for tetrazolium ions. Molecular electrostatic potential (MEP) calculations also indicate that tetrazolium ions possess two sites for proton hopping. This makes such materials interesting for use in a high temperature fuel cell (HT PEMFC). Based on these findings, two polymers incorporating the proposed TZ groups were synthesised, formed into membranes, doped with PA and tested for fuel cell relevant properties. At room temperature, TZ-PEEN and commercial meta-PBI showed an equilibrium uptake of 0.5 and 4.7 mol PA per mol heterocycle, respectively, indicating that PBI has higher affinity for PA than TZ-PEEN. The highest achieved PA uptake was ca. 110 wt%, resulting in a proton conductivity of 25 mS cm−1 at 160 °C with a low activation energy of about 35 kJ mol−1. In a first HT PEMFC test at 160 °C, a peak power density of 287 mW cm−2 was achieved.


 Please wait while we load your content...
Please wait while we load your content...