Nitrogen-rich energetic 4-R-5-nitro-1,2,3-triazolate salts (R = –CH3, –NH2, –N3, –NO2 and –NHNO2) as high performance energetic materials†
Abstract
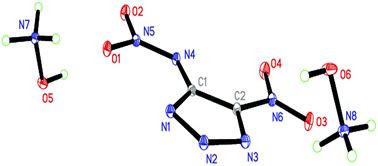
A series of 4-R-5-nitro-1,2,3-triazolate salts (R = –CH3, –NH2, –NO2, –N3, and –NHNO2) were synthesized with cations of ammonium, hydroxylammonium, hydrazinium, guanidinium, aminoguanidinium, diaminoguanidinium, and triaminoguanidinium. The resulting energetic salts were characterized by 1H and 13C NMR, IR spectroscopy, elemental analysis, and in some cases by single crystal X-ray diffraction. Their key properties were measured or calculated such as melting and decomposition temperatures, density, detonation pressure and velocity, and impact and friction sensitivities. The results show that guanidinium salts possess the highest thermal stability in each group with decomposition temperatures of 265, 251, 221, 142, and 216 °C, respectively. Hydroxylammonium 4,5-dinitro-1,2,3-triazolate and dihydroxylammonium 4-nitramino-5-nitro-1,2,3-triazolate exhibit much higher detonation performances (38.0 GPa and 9302 m s−1; 38.8 GPa and 9464 m s−1) than cyclotrimethylenetrinitramine (34.9 GPa and 8748 m s−1). Moreover, the high specific impulses (256.1 and 251.1 s) from the formula calculations based on the two salts further support their application prospects.

 Please wait while we load your content...
Please wait while we load your content...