Vertically aligned VO2(B) nanobelt forest and its three-dimensional structure on oriented graphene for energy storage†
Abstract
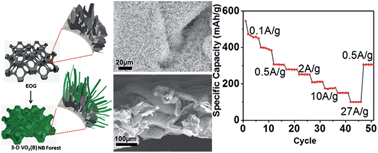
Assembling two-dimensional (2D) nanomaterials into an ordered forest structure that provides an easily accessible large surface area and/or chemically active 2D edges will present numerous opportunities, including as electrodes for energy storage. We report a densely packed vertically aligned VO2(B) nanobelt (NB) based forest structure synthesized by a solvothermal method using a vertically oriented graphene (VOG) network as the underlying support. We further expanded this forest structure into a folded three-dimensional (3D) forest structure using VOG-covered metallic foam as the scaffold. To demonstrate its potential, this free-standing 3D ordered structure built from 2D nanomaterials was directly used as the electrode for lithium-ion batteries. Excellent performance was confirmed by a stable discharge capacity of 178 mA h g−1 at a current density of 10 A g−1 (or a rate of 59 C) and 100 mA h g−1 at 27 A g−1 (300 C), contributed by both lithium ion intercalation into the crystal lattice and surface-related pseudocapacitance. A high cycling stability over 2000 cycles under a high current density was also demonstrated. We expect that our method can be expanded to synthesize 2D sheet based forest structures of other layered oxides and hydroxides by using VOG as a versatile platform for numerous applications such as energy storage and catalytic energy conversion.

 Please wait while we load your content...
Please wait while we load your content...