Lithium transport through lithium-ion battery cathode coatings
Abstract
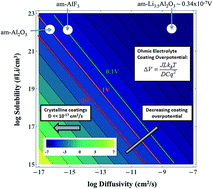
The surface coating of cathodes using insulator films has proven to be a promising method for high-voltage cathode stabilization in Li-ion batteries, but there is still substantial uncertainty about how these films function. More specifically, there is limited knowledge of lithium solubility and transport through the films, which is important for coating design and development. This study uses first-principles calculations based on density functional theory to examine the diffusivity of interstitial lithium in the crystals of α-AlF3, α-Al2O3, m-ZrO2, c-MgO, and α-quartz SiO2, which provide benchmark cases for further understanding of insulator coatings in general. In addition, we propose an ohmic electrolyte model to predict resistivities and overpotential contributions under battery operating conditions. For the crystalline materials considered we predict that Li+ diffuses quite slowly, with a migration barrier larger than 0.9 eV in all crystalline materials except α-quartz SiO2, which is predicted to have a migration barrier of 0.276 eV along 〈001〉. These results suggest that the stable crystalline forms of these insulator materials, except for oriented α-quartz SiO2, are not practical for conformal cathode coatings. Amorphous Al2O3 and AlF3 have higher Li+ diffusivities than their crystalline counterparts. Our predicted amorphous Al2O3 resistivity (1789 MΩ m) is close to the top of the range of the fitted resistivities extracted from previous experiments on nominal Al2O3 coatings (7.8 to 913 MΩ m) while our predicted amorphous AlF3 resistivity (114 MΩ m) is very close to the middle of the range. These comparisons support our framework for modeling and understanding the impact on overpotential of conformal coatings in terms of their fundamental thermodynamic and kinetic properties, and support that these materials can provide practical conformal coatings in their amorphous form.

 Please wait while we load your content...
Please wait while we load your content...