The solid-state chelation synthesis of LiNi1/3Co1/3Mn1/3O2 as a cathode material for lithium-ion batteries†
Abstract
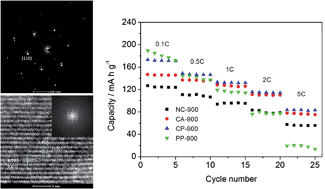
A facile solid-state chelation method using citric acid as the solid chelant was investigated for the synthesis of layered LiNi1/3Co1/3Mn1/3O2 as a cathode material for rechargeable lithium-ion batteries. The reaction was promoted by high-energy ball milling. During the synthesis, PVP was used as an additive. For comparison, LiNi1/3Co1/3Mn1/3O2 was also synthesized by a conventional sol–gel method using citric acid as the chelant. The as-prepared samples were characterized by TG-DSC, XRD, FESEM, BET specific surface area and galvanostatic charge–discharge tests. Based on the XPS, TEM and ED results, the sample synthesized by the solid-state chelation method with the PVP as an additive and subsequent calcination at 900 °C for 12 h in air was well indexed to a pure-phase hexagonal α-NaFeO2 structure with the highest crystallinity. The resulting sample showed an initial discharge capacity of 173 mA h g−1 in the potential range of 2.6–4.5 V and at a rate of 0.1 C, higher than that of the sample prepared by the same method without the use of a PVP additive during the synthesis (146 mA h g−1). Moreover, the electrochemical results at different current rates and the cycle performance for 100 cycles at 0.5 C indicated that the sample prepared by the solid-state chelation method exhibited better rate capability and cyclic stability than that prepared by the conventional sol–gel method. This phenomenon promises solid-state chelation as a new universal method for the preparation of functional materials.

 Please wait while we load your content...
Please wait while we load your content...