Improved environmental stability of organic lead trihalide perovskite-based photoactive-layers in the presence of mesoporous TiO2†
Abstract
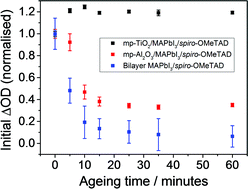
Impressive hybrid photovoltaic device performances have been realised with the methylammonium lead triiodide (MAPbI3) perovskite absorber in a wide range of device architectures. However, the question as to which of these systems represents the most commercially viable long-term prospect is yet to be answered conclusively. Here, we report on the photoinduced charge transfer processes in MAPbI3 based films measured under inert and ambient conditions. When exposed to ambient conditions, the coated mesoporous Al2O3 and bilayer systems show a rapid and significant degradation in the yield of long-lived charge separation. This process, which does not affect sensitized-mesoporous TiO2 films, is only found to occur when both light and oxygen are present. These observations indicate that the presence of a mesostructured TiO2 electron acceptor to rapidly extract the photoexcited electron from the perovskite sensitizer may be crucial for fundamental photovoltaic stability and significantly increases innate tolerance to environmental conditions. This work highlights a significant advantage of retaining mesoscale morphological control in the design of perovskite photovoltaics.
- This article is part of the themed collection: Highlighting materials research in the UK for energy and sustainability

 Please wait while we load your content...
Please wait while we load your content...