Ultrathin TiO2-B nanowires with enhanced electrochemical performance for Li-ion batteries†
Abstract
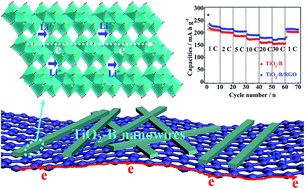
A facile one-step hydrothermal route was designed for preparing ultrathin TiO2-B nanowires, which were then hybridized with RGO to form a TiO2-B/RGO hybrid via an in situ approach, and both of them have a large BET surface area (231.6 m2 g−1 for TiO2-B nanowires and 256.1 m2 g−1 for the TiO2-B/RGO hybrid). It was found that the synthesized ultrathin nanowires are perpendicular to the [010] direction which is the most open channel in the TiO2-B crystal structure, demonstrating more Li-ion insertion/extraction hosts exposed to the electrolyte. Thus, the cell made of TiO2-B ultrathin nanowires exhibited large reversible lithium-ion charge–discharge capacity, excellent cycling stability and high-rate capability. When combined with RGO, the formed TiO2-B/RGO hybrid exhibited further improved Li storage performance. For instance, a capacity of 205.3 mA h g−1 was obtained at the fourth cycle and then faded slightly to 189.4 mA h g−1 after 300 cycles, demonstrating a surprising low average capacity fading of ca. 0.026% per cycle from 4th to 300th cycles.

 Please wait while we load your content...
Please wait while we load your content...