The origins and mechanism of phase transformation in bulk Li2MnO3: first-principles calculations and experimental studies†
Abstract
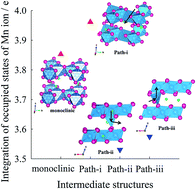
Lithium-rich oxide materials are promising candidates for high-energy lithium ion batteries, but currently have critical challenges of poor cycle performance and voltage drop induced by undesirable phase transformation. To resolve these problems, it is necessary to identify the origins and mechanism of phase transformation in Li2MnO3, a key component of Li-rich oxides. In this work, the phase transformation of bulk Li2MnO3 is investigated by thermodynamic and kinetic approaches based on first-principles calculations and validated by experiments. Using the calculated thermodynamic energies, the most stable structure is determined as a function of Li extraction for Li2−xMnO3: monoclinic (x = 0.0–0.75), layered-like (x = 1.0–1.25), and spinel-like (x = 1.5–2.0) structures. The phase transformation becomes kinetically possible for Li2−xMnO3 (x > 1.0). Atomic scale origins and the mechanism of phase transformation are elucidated by the thermodynamically stable and kinetically movable tetrahedral coordination of Mn4+ in the transition state. These theoretical observations are validated by ex situ X-ray photoelectron spectroscopy combined with electrochemical experiments for Li2−xMnO3 with various Li contents upon cycling. The mechanistic understanding from theoretical calculations and experimental observations is expected to provide a fundamental solution and guidelines for improving the electrochemical performance of Li-rich oxides and, by extension, the battery performance.

 Please wait while we load your content...
Please wait while we load your content...