Persulfate salt as an oxidizer for biocidal energetic nano-thermites†
Abstract
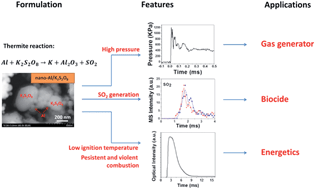
Nanoscale potassium persulfate (K2S2O8) was evaluated as an alternative to other peroxy salts, such as periodates (KIO4), in aluminum-fueled energetic nano-composite formulations. High speed imaging coupled with temperature jump (T-jump) ignition found the nano-Al/K2S2O8 reaction to have an ignition temperature of 600 °C which is comparable to nano-Al/KIO4 and lower than nano-Al/K2SO4. The results from constant-volume pressure cell experiments further show that nano-Al/K2S2O8 releases more gas and has a longer burn time than nano-Al/KIO4. Thermal analyses at low heating rates (10 °C min−1) by coupled differential scanning calorimetry (DSC), thermal gravimetric analysis (TG) and mass spectrometry (MS) show that there are three main steps of thermal decomposition for nano-K2S2O8, with initial exothermic decomposition to release O2 at 270 °C, and following endothermic decomposition to release both O2 and SO2 at higher temperatures. The heat of formation of K2S2O8 was measured to be −1844.5 kJ mol−1 based on the DSC results. Experiments performed at ultrafast heating rates (∼105 °C s−1) using temperature-jump time-of-flight (T-jump/TOF) MS show that the low O2 generation temperature of nano-K2S2O8 contributes to its high reactivity in nano-thermite compositions. An ignition mechanism involving gaseous oxygen was proposed for nano-thermite compositions containing reactive oxysalts such as nano-K2S2O8. In contrast, a condense phase ignition mechanism was proposed for nano-thermites involving less reactive oxysalts such as nano-K2SO4. Given that the nano-Al/K2S2O8 system is highly exothermic in addition to generating a considerable amount of SO2, it may be a candidate for use in energetic biocidal applications.


 Please wait while we load your content...
Please wait while we load your content...