Sodiation vs. lithiation phase transformations in a high rate – high stability SnO2 in carbon nanocomposite†
Abstract
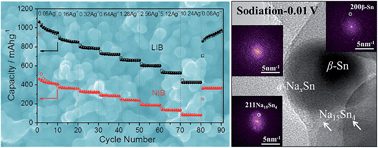
We employed a glucose mediated hydrothermal self-assembly method to create a SnO2–carbon nanocomposite with promising electrochemical performance as both a sodium and a lithium ion battery anode (NIBs NABs SIBs, LIBs), being among the best in terms of cyclability and rate capability when tested against Na. In parallel we provide a systematic side-by-side comparison of the sodiation vs. lithiation phase transformations in nano SnO2. The high surface area (338 m2 g−1) electrode is named C–SnO2, and consists of a continuous Li and Na active carbon frame with internally imbedded sub-5 nm SnO2 crystallites of high mass loading (60 wt%). The frame imparts excellent electrical conductivity to the electrode, allows for rapid diffusion of Na and Li ions, and carries the sodiation/lithiation stresses while preventing cycling-induced agglomeration of the individual crystals. C–SnO2 employed as a NIB anode displays a reversible capacity of 531 mA h g−1 (at 0.08 A g−1) with 81% capacity retention after 200 cycles, while capacities of 240, 188 and 133 mA h g−1 are achieved at the much higher rates of 1.3, 2.6 and 5 A g−1. As a LIB anode C–SnO2 maintains a capacity of 1367 mA h g−1 (at 0.5 A g−1) after 400 cycles, and 420 mA h g−1 at 10 A g−1. Combined TEM, XRD and XPS prove that the much lower capacity of SnO2 as a NIB anode is due to the kinetic difficulty of the Na–Sn alloying reaction to reach the terminal Na15Sn4 intermetallic, whereas for Li–Sn the Li22Sn5 intermetallic is readily formed at 0.01 V. Rather, with applied voltage a significant portion of the material effectively shuffles between SnO2 and β-Sn + NaO2. The conversion reaction proceeds differently in the two systems: LiO2 is reduced directly to SnO2 and Li, whereas the NaO2 to SnO2 reaction proceeds through an intermediate SnO phase.

 Please wait while we load your content...
Please wait while we load your content...