Effect of the oxidation approach on carbon nanotube surface functional groups and electrooxidative filtration performance†
Abstract
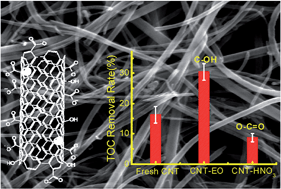
Carbon nanotubes (CNTs) have found utility as an anode material for contaminant degradation; however, anode materials will inevitably undergo surface oxidation that in general has negative effects on the electrochemical performance. Here, we observe that the specific CNT surface oxidation approach can preferentially form specific surface oxy-functional groups that in turn can significantly affect the CNT anode performance. CNT anodes were oxidized by two methods; (1) preanodization in a sodium sulfate electrolyte (CNT–EO) to simulate electrochemical oxidation of CNT anodes, and (2) reflux in nitric acid (CNT–HNO3) for comparison with CNT–EO. CNT characterization by XPS, FT-IR, and Boehm titration indicated that the unmodified CNT surface oxy-groups were 35.9% hydroxyl (–COH) and 64.1% carboxyl (–COOH), the CNT–EO surface groups were 47.8% –COH and 52.2% –COOH, and the CNT–HNO3 surface groups were predominantly –COOH. As compared to an unmodified CNT anode control, the CNT–EO performance was increased by 2-fold and the CNT–HNO3 performance was decreased by 2-fold in regards to anodic phenol mineralization and a similar trend was observed for oxalate mineralization. In agreement with the increased CNT–EO anodic performance, cyclic voltammetry and electrochemical impedance spectroscopy indicated that the CNT–EO had a lower phenol electrooxidation overpotential and lower resistance to charge transfer, respectively, than both the fresh CNT and CNT–HNO3. Thus, the specific CNT surface oxy-functional group has a strong effect on anodic performance. To further explore the effect of specific CNT surface groups on the electrooxidation performance, experiments were also completed for the classical probe molecules of potassium ferricyanide and ascorbic acid (AA).

 Please wait while we load your content...
Please wait while we load your content...