Formation of bilayer clathrate hydrates†
Abstract
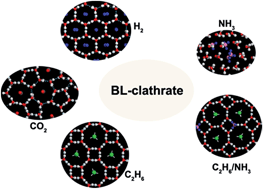
We report molecular dynamics (MD) simulation evidence for a new family of two-dimensional (2D) clathrate hydrates. Particular attention is placed on the effect of the size and hydrophilicity of guest-molecules on the formation of 2D clathrate hydrates. Among the MD simulations undertaken, the spontaneous formation of bilayer (BL) clathrate hydrates in nanoslits are found with five different hydrophobic guest molecules, namely, ethane (C2H6), ethene (C2H4), allene (C3H4), carbon dioxide (CO2) and hydrogen (H2) molecules. Our simulations suggest that the host cages in a water framework are likely BL-hexagonal cages with single occupancy for H2 or BL-heptagonal cages for CO2. With a further increase in guest size, the host cages for C2H6, C2H4, and C3H4 are BL-octagonal cages with single occupancy, and their long molecular axis tends to be normal to the surface of clathrate hydrates. In addition, for hydrophilic guest molecules, such as NH3 and H2S, which can form strong hydrogen bonds with water, we find that most guest molecules can preferentially displace water molecules from the lattice sites of the water framework, instead of being separately trapped within the water cages. Structural analogy between the 2D and 3D clathrates enlightens us to predict the stability of several bulk gas hydrates, namely, “ethane clathrate III”, “CH4 ice-i” and “H2 ice-i”. Our findings can not only enrich clathrate structures in the hydrate family but may also improve the understanding of hydrate formation in microporous media.

 Please wait while we load your content...
Please wait while we load your content...