A thin film sorbent of layered organo-MnO2 for the extraction of p-aminoazobenzene from aqueous solution†
Abstract
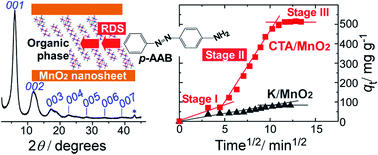
An electrochemically grown thin film sorbent, consisting of layered manganese dioxide (MnO2) intercalated with cetyltrimethylammonium (CTA) cations, was employed to remove p-aminoazobenzene (p-AAB), a neutral organic dye, from aqueous solution. The CTA+-intercalated MnO2 (CTA/MnO2) film sorbed approximately six times more p-AAB than a film of K+-intercalated MnO2 (K/MnO2). The equilibrium p-AAB sorption data obtained for the CTA/MnO2 film exhibited a better fit to the Langmuir isotherm than the Freundlich isotherm. The maximum sorption capacity of the film was determined to be 781 mg p-AAB per gram of electrodeposited MnO2. The sorption of p-AAB was not affected by the presence of an excess of small ions such as Na+ and SO42−. XRD and FTIR analyses demonstrated that the p-AAB molecules were accommodated in large, surfactant-filled interlayer spaces between MnO2 layers, and that the layered structure was maintained during sorption. The versatility of this sorbent was verified by its high sorption capacities for cationic (methylene blue) and anionic (Congo red) dyes. The sorption kinetics were well described by an intra-particle diffusion model, rather than by pseudo-first-order or pseudo-second-order models, and diffusion into the interlayer spaces was found to be the rate controlling step over a wide range of contact times, where a rate constant of 73.6 mg g−1 min−1/2 was estimated. The p-AAB molecules sorbed in the interlayer spaces were gradually expelled after immersing the film in a solution containing solely the supporting electrolyte. When the film was polarized at 0 V vs. Ag/AgCl, the p-AAB desorption was remarkably accelerated as the result of the expulsion of intercalated CTA molecules upon reduction of the MnO2 layers due to charge compensation.

 Please wait while we load your content...
Please wait while we load your content...