Eugenol-based benzoxazine: from straight synthesis to taming of the network properties†
Abstract
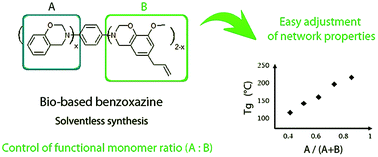
Mixed benzoxazine precursors were synthesized using a blend of eugenol, a natural renewable product, and phenol. The structures of these mixed benzoxazine precursors with different phenol : eugenol compositions were attested by 1H NMR. Their polymerization and degradation were investigated and monitored by DSC and TGA and showed an enhancement of the crosslinking ability with the phenol content. Depending on its relative content, the phenol moiety proved to limit the thermal degradation of the bis-benzoxazine “hybrid” monomer and allowed the formation of crosslinked networks with high thermomechanical stabilities. The properties of the networks were closely dependent on the phenol : eugenol ratio, which allowed for adjustment of the crosslinking density and fine tuning of the glass transition temperature (Tg) within a wide temperature range. A comparison between the polymerized hybrid precursors and blend of two pure monomers displaying the same overall composition showed the same material properties increasing the tunability of the system. The eugenol/phenol combination for the preparation of mixed/hybrid benzoxazines or corresponding blends clearly paves the way to new sustainable high performance bio-based materials.

 Please wait while we load your content...
Please wait while we load your content...