Activated carbon-based gas sensors: effects of surface features on the sensing mechanism†
Abstract
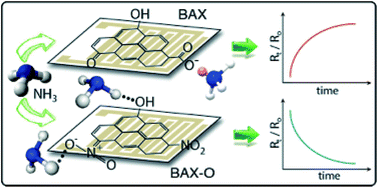
Ammonia sensing capability of a commercial wood-based activated carbon (BAX) and its oxidized counterpart (BAX-O) was studied. The materials were exposed to continuous cycles of various ammonia concentrations, followed by purging with air, and changes in a normalized resistance were analyzed. When initially exposed to the reducing gas, chemical interactions between ammonia and the carbons generated an irreversible signal change, which was stronger in the case of the initial carbon. After equilibration and reactive adsorption the sensors were further tested with ammonia. During the reversible sensing, the two carbons exhibited opposite trends in their signal. This indicates that oxidation changed the electrical properties of the carbon sample, leading to a different sensing mechanism. For the initial material holes play the predominant role in a current transport, while their depletion upon the exposure to the reducing gas leads to a decrease in the conductivity. In the case of the oxidized sample, the presence of electron donating nitro groups causes a hole annihilation in the carbon itself converting it to a predominantly n-type material. Here the exposure to ammonia increases the conductivity of the chip owing to the electron donating properties of the gas. Physical interactions, such as hydrogen bonding, polar interactions with surface functional groups, and dispersive interactions of ammonia with the carbon matrix, lead to the pore filling, which governs the extent of the response signal. The sensor signal changes linearly with the ammonia concentration.

 Please wait while we load your content...
Please wait while we load your content...