A study of the effect of the addition of nanostructured carbonaceous catalysts in the dehydrogenation mechanism of magnesium borohydride
Abstract
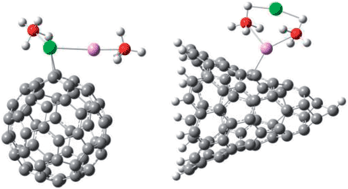
In this work first principles calculations based on density functional theory are performed to study the effect of using carbon fullerenes C60 (buckyballs) or C67 (nanohorns) as catalyzing agents in the dehydrogenation of magnesium borohydride. This study focuses on thermodynamic quantities, i.e., the energy changes following H-removal with and without the catalysts. The calculated energy cost for H-removal from Mg(BH4)2 is 4.18 eV, which decreases to 4.08 eV when adding C60 and to 3.32 eV in the presence of C67. With a combined addition of titanium besides the carbonaceous catalysts the H-removal energy cost decreases to 4.04 eV and 2.78 eV, in the presence of C60 and C67, respectively. This arises from the weakening of the B–H bonds which takes place through an interplay mechanism of charge transfer between the Mg(BH4)2 cluster and the catalysts.

 Please wait while we load your content...
Please wait while we load your content...