Oxygen surface exchange kinetics and stability of (La,Sr)2CoO4±δ/La1−xSrxMO3−δ (M = Co and Fe) hetero-interfaces at intermediate temperatures†
Abstract
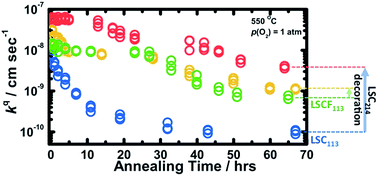
Heterostructured oxide interfaces created by decorating Ruddlesden–Popper (RP) phases on ABO3 perovskites have shown not only pronounced cation segregation at the interface and in the RP phase but also enhanced kinetics for oxygen electrocatalysis at elevated temperatures. In this study, combining experimental and theoretical approaches, we report and compare the time-dependent surface exchange kinetics and stability of (La0.5Sr0.5)2CoO4±δ (LSC214)-decorated La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF113) and La0.8Sr0.2CoO3−δ (LSC113) thin films. While LSC214 decoration on LSC113 greatly reduced the degradation in the surface exchange kinetics as a function of time relative to undecorated LSC113, LSCF113 with LSC214 coverage showed comparable surface exchange kinetics and stability relative to undecorated LSCF113. This difference is attributed to stabilization of the LSC113 surface by LSC214 decoration and greater stability of LSCF113 against decomposition into secondary phases than LSC113. This hypothesis is supported by density functional theory (DFT) computation, revealing greater surface Sr segregation for LSCF113, which is predicted to have an SrO termination, than LSC113, which is predicted to have a less Sr enriched (La0.25Sr0.75)O termination. Furthermore, DFT also showed a lower energy gain to move Sr from LSCF113 into LSC214 relative to the LSC214-LSC113 surface, and predicted the stability of LSCF113, LSC113, and LSC214 with 100% Sr substitution in their top (001) surface. The stability differences of Sr substitution (with La) in LSCF113, LSC113, and LSC214, along with the assessed DFT decomposition free energies of fully Sr substituted LSCF113, LSC113, and LSC214, correlate with the experimental observation of surface stability trends in surface particle formation of LSCF113 and LSC113 without and with LSC214 decoration.

 Please wait while we load your content...
Please wait while we load your content...