A new magnetic nanocomposite for selective detection and removal of trace copper ions from water†
Abstract
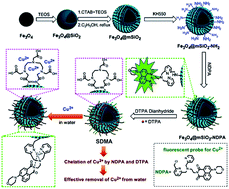
A core–shell structured, magnetic nanocomposite (SDMA) modified by a new organic fluorescent probe and selective chelating groups was prepared for simultaneous detection and removal of low Cu2+ concentrations. A series of experiments was designed to detect and adsorb copper ions in aqueous solution via SDMA. Results showed that SDMA could detect Cu2+ from copper ion solution qualitatively and quantifiably with a certain degree of selectivity, and remove Cu2+ with a respectable removal efficiency of about 80%. In comparative adsorption experiments, the adsorption capacity of SDMA was significantly higher than that of another two common magnetic nanoadsorbents (Fe3O4@mSiO2–SH and Fe3O4@mSiO2–NH2). The adsorption behavior of SDMA was studied through equilibrium and kinetic experiments. The adsorption isotherm was perfectly fitted via the Freundlich model and the pseudo-second-order model could fit the kinetic adsorption. Moreover, the SDMA could reach the adsorption equilibrium in only 20 min, which showed a fast kinetic adsorption to Cu2+. Prepared SDMA can be an effective and potential nanoadsorbent for detecting and removing copper ions from wastewater.

 Please wait while we load your content...
Please wait while we load your content...