Anisotropic oxide ion conduction in melilite intermediate temperature electrolytes†
Abstract
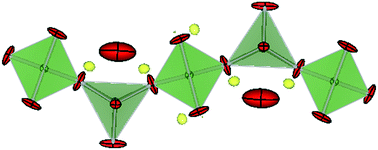
Electrolytes with oxide ion conductivities higher than 10−2 S cm−1 at moderate temperatures (∼500–900 °C) offer the possibility for solid oxide fuel cells to operate with less maintenance. This study of [A1+xB1−x]2[Ga]2[Ga2O7+x/2]2 (0 ≤ x ≤ 0.5) (A = La, Nd; B = Ca, Sr) layered-melilite found that in large single crystals intralayer oxide ion conduction is dominant. This anisotropic behavior arises by relaxation about the interstitial oxygen through changes in the interlayer A and Ga coordination, and at 850 °C conductivities are ∼0.008 S cm−1 along the c direction and ∼0.036 S cm−1 perpendicular to the c axis. It is found that the ionic conductivity can be optimized by increasing the number of interstitial oxygen and reducing the size of interlayer cations.

 Please wait while we load your content...
Please wait while we load your content...