Tailoring mixed ionic–electronic conduction in H2 permeable membranes based on the system Nd5.5W1−xMoxO11.25−δ†
Abstract
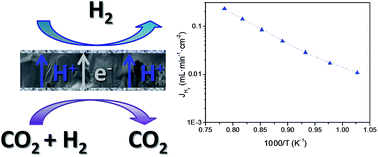
Lanthanide tungstates (Ln6WO12) are promising candidates for the development of ceramic hydrogen transport membranes since they exhibit mixed ionic (proton and oxygen ion transport) and electronic conductivity and remarkable stability in a moist CO2 environment at high temperatures. This work presents the structural and electrochemical characterization of mixed conducting materials for the specific system Nd5.5W1−xMoxO11.25−δ (x = 0, 0.1, 0.5 and 1). Evolution of the crystalline structure is studied as a function of the sintering temperature. Shrinkage behavior is analyzed for all compositions in the temperature range from 1000 °C to 1500 °C and these compounds show high sintering activity even at relatively low temperatures. The total conductivity in different environments is studied systematically for samples sintered at 1350 °C. The H/D isotopic effect is also studied by DC-electrochemical measurements. H2 permeation is investigated for the selected compound Nd5.5W0.5Mo0.5O11.25−δ in the range of 700–1000 °C achieving values of 0.3 mL min−1 cm−2 for a 0.9 mm thick disc membrane. Finally, the stability of this material under different CO2 and H2S-rich atmospheres at high temperatures is proven.

 Please wait while we load your content...
Please wait while we load your content...